About us.
We are headquartered in Singapore with wholly-owned subsidiaries in Basel, Switzerland, and Sydney, Australia.
What we do
We are changing the status quo of delivery of various drug payloads to significantly enhance their efficacy. We have spent over 15 years on this journey, perfecting our modular and scalable processes.
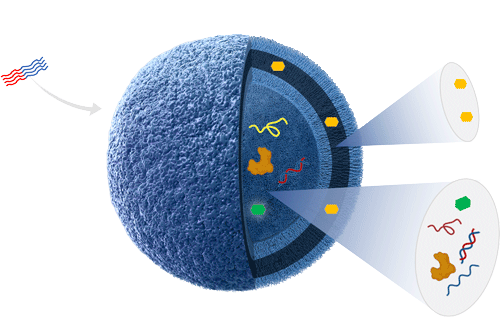
Our patent protected ACM Biolabs’ Tunable Platform (ATPTM) is a polymer/lipid hybrid, non-viral delivery technology that offers the flexibility to deliver multiple payloads including oligonucleotides, small molecules, proteins, and mRNA.
In our CEPI-funded mRNA rabies vaccine program, ATPTM nanoparticles keeps mRNA stable at 2-8°C in contrast to the current lipid nanoparticle-based delivery systems that require ultra-low cold storage. This critical improvement allows use of such vaccines in countries that struggle to access mRNA vaccines due to a lack of an established cold-chain infrastructure.
In our oncology program, ATPTM nanoparticles have been shown to fundamentally alter the mechanism of action of their CpG oligonucleotide cargo, enabling myeloid modulation through comprehensive TLR9 engagement in immunologically cold tumors. The anticipated efficacy enhancements are being borne out by early clinical readouts.
The Team.
Chairman of the Board ACM Biolabs & ACM Biosciences
Business Development
Developed the core intellectual property of the company while working in Germany, the Netherlands and Singapore (at the Institute of Materials Research & Engineering (IMRE, A*STAR) and also at Nanyang Technological University).


Pharmacologist. Vaccines Expert with more than 30 years of experience of R&D.
Doctorat at the Université catholique de Louvain, Belgium. Leading function at GSK, Abivax, NeoVacs, Imcyse, and Amyl Therapeutics. CEO at Vaccibio Consulting.

Founder of the Erber Group, an Austrian animal nutrition company (now part of DSM), which consists of more than 50 affiliates in all continents. He is also founder of the SAN Pacific Investments Group. Recipient of numerous awards including the Austrian Export award and Ernst & Young Entrepreneur of the Year and is an Honorary Senator of the University of Veterinary Medicine and the University of Natural Resources and Applied Life Sciences, Vienna.
Erich is the key investor representative on ACM Biolabs’ Board of Directors.

Sang Bin is currently the managing director of Ritz Venture Capital, a Singapore based family fund that focuses in supporting early-stage companies. He has over 30 years of experience in venture investments as well as mentorship within the academic community. With his background in engineering and business, he is a strong supporter of applying cutting edge technology and cultivating talents to address some of the key challenges in the world in the near future.

Maria leverages 15 years’ experience as founder and CEO of an ASX listed biotechnology company, and a prior 10 years in corporate finance and venture capital, assisting early-stage companies with capital raising, scale -up and international expansion. Recognised for her experience in funding and commercialising technologies internationally, Maria executed several cross-border mergers and acquisitions, opened new markets, built multidisciplinary teams, led digital transformation and navigated companies through complex regulatory environments. An experienced non-executive director and contributor to the start-up ecosystem, Maria is a Graduate of the University of Western Australia and the Australian Institute of Company Directors, she is a Non-executive Director of the Garvan Research Foundation, Member of Women on Boards, Ausbiotech and Women in Media, Connector at Apropela and a past Councilor at the Australian Industry Group. A lifelong running enthusiast Maria has a keen interest in lifespan expansion and healthy longevity and is passionate about taking others along a healthy longevity journey.


PhD. Managed the Micro- and Nano-systems group of 50 R&D staff in the Singapore research institute where the polymersome technology originated. Thereafter, co-founded Singular ID, a successful tech start-up which received funding from institutional investors based in the US, Europe and Asia and was acquired by a multinational. Has worked with Dr Nallani on ACM since 2011.

Katherine Schultheis joined ACM Biosciences in 2022 as Head of Discovery Research with a strong background of preclinical development of DNA based vaccines and immunotherapeutics. Katherine has more than 10 years of academic and corporate research experience in the fields of infectious diseases, autoimmunity and oncology. She was heading a research team at Inovio Pharmaceuticals in San Diego, California, before moving back to the greater Basel area in 2021. Katherine obtained her Master’s level degree in Biochemistry from University of Potsdam, Germany.


Before joining ACM Biolabs, Qiwei was an audit manager with experience in SMEs and subsidiaries of MNCs of various industries. He is also a member of the Institute of Singapore Chartered Accountants and CPA Australia.

Prior to joining ACM Biolabs, Melvin received his PhD in Materials Science and Engineering from Nanyang Technological University in 2023. His PhD research focused on the fabrication and characterization of biomimetic nanoparticles for drug and RNA delivery.

Dr. Liu received her PhD in chemical and biomolecular engineering from National University of Singapore (NUS). She has more than 15 years of research and development experience in Biomedical science and Bioengineering. She has generated 5 patents and 42 papers in reputable journals including Nature communications and Advanced Materials.

Jian Hang Lam holds a Ph.D. from the Yong Loo Lin School of Medicine, National University of Singapore. He was trained in pre-clinical dengue vaccine development and gained experience in virology and immunology, with special focus on investigating correlates of protection.

Ser Yue Loo is a Senior Scientist at ACM Biolabs. Prior to joining ACM Biolabs, Ser Yue carried out her postdoctoral fellowship at Genome Institute of Singapore, where she conducted research on therapeutics for treatment of triple negative breast cancer. Her work led to the successful translation from bench to bedside with the initiation of a clinical trial BEXMET in National Cancer Centre Singapore.
Loo Ser Yue obtained her PhD from Yong Loo Lin School of Medicine at National University of Singapore in 2014.

Gaurav joined ACM Biolabs in 2021, after completing his PhD research in the field of point-of-care diagnostics from the School of Material Science and Engineering at NTU.

Yan Jun Lee is a Scientist at ACM Biolabs. She obtained her PhD from Nanyang Technological University, Singapore in 2021. She then carried out her postdoctoral fellowship at National University of Singapore. She has conducted neuroscience studies in molecular and system settings throughout her academics training.

Graduated from Nanyang Technological University in 2019 with a Bachelor’s Degree in Chemistry and Biological Chemistry. Joined ACM Biolabs in 2019 as a Research Assistant.

Research Assistant at ACM Biolabs with a Bachelor of Engineering in Bioengineering obtained in 2024 from Nanyang Technological University.

Research Assistant at ACM Biolabs. Obtained a diploma in Chemical Engineering in 2015 and Bachelor’s Degree in Chemistry and Biological Chemistry in 2021 at Nanyang Technological University.

Research Assistant at ACM Biolabs. Obtained a diploma in Medicinal Chemistry in 2017 and a degree of Bachelor of Science in Chemistry and Biological Chemistry in 2021 at Nanyang Technological University.”

We are looking for highly motivated specialists – take a look at our career offers
Technology Platform.
Pipeline.
A growing pipeline comprising targeted delivery of TLR agonists as vaccines and therapies against infectious diseases, respiratory infections and cancer.
At ACM Biolabs we are driven to change the status quo necessitated by the limitations of current delivery technologies. We are focused on leveraging the strengths of the ATP™ technology to improve the effectiveness of TLR agonists against diverse illnesses. Thereafter we will broaden the focus to other modalities.
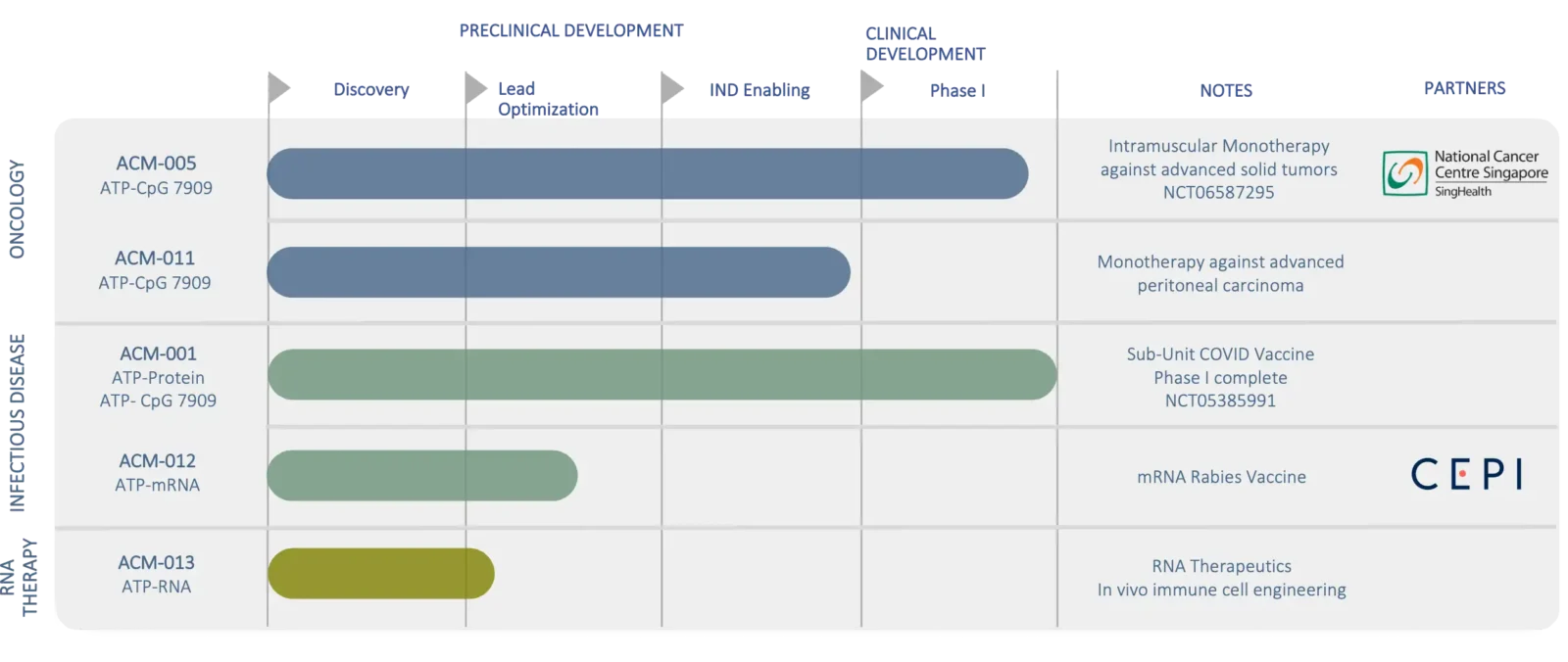
* Platform Clinical Validation
** IIT with National Cancer Center Singapore (NCCS)
Documented achievements.
News.
Contact.
bd@acmbiolabs.com
office@acmbiosciences.com
ACM Biolabs Pte Ltd
Phone: +65 6265 5646
ACM Biolabs Pty Ltd
139 Macquarie StreetSydney NSW 2000
Australia
ACM Biosciences AG
4052 Basel
Switzerland
Career Opportunities
We are currently building a high quality and motivated team to develop our polymer-based delivery technology and bring innovative medicines to patients with high unmet medical need. Working at the intersection of pre-clinical and clinical research as a part of multidisciplinary team We we are looking for candidates with expertise in mRNA technologies, nano-technology, chemistry of lipids- and polymer-based delivery formulations and analytical methods. Interested applicants should please forward their resumé / CV to career@acmbiolabs.com